Quality and Safety Initiatives for Pigeon Products: Providing Customers with Safe and Reliable Products
Approach to Quality Assurance
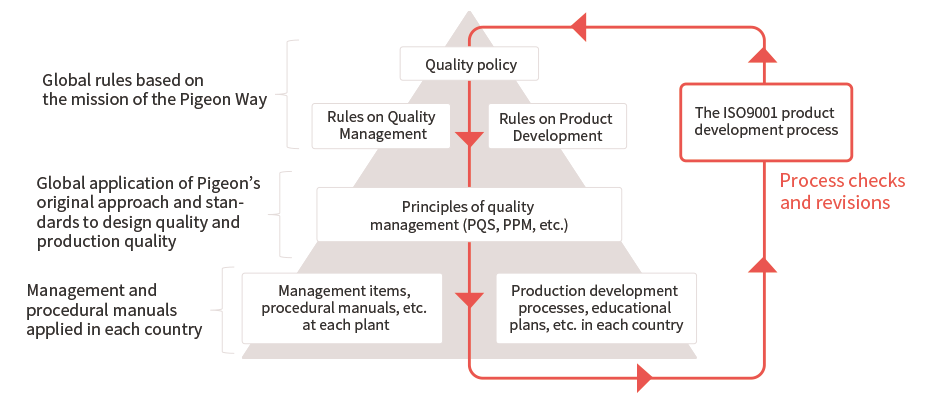
The Pigeon Group is passionate about providing safe and reliable products to babies and their families all over the world. To accomplish this task, the Group has built an original quality assurance system that improves quality in two stages: Design quality, at the product-development stage, and production quality, at the manufacturing stage.
To support and improve Design Quality, the Pigeon Group specifies the Pigeon Quality Standards (PQS). These standards embody an original product-design approach and criteria that build safety, ease of use and durability into products from the product-development stage onward. By applying PQS globally, we are working to support and improve the design quality of our products manufactured worldwide.
In production, we strive to achieve stable quality through key standards and practices. One of these is Pigeon Productive Management (PPM), our original set of criteria for the manufacturing equipment and production environments at each plant. Another is Good Manufacturing Practice (GMP), established for each country. We also conduct regular plant audits, among other activities.
In these two initiatives to improve quality, the processes are managed according to ISO 9001. The measures are checked and revised under this international standard every six months.
Quality Assurance System
Our Safety Criteria (for Safe Manufacturing)
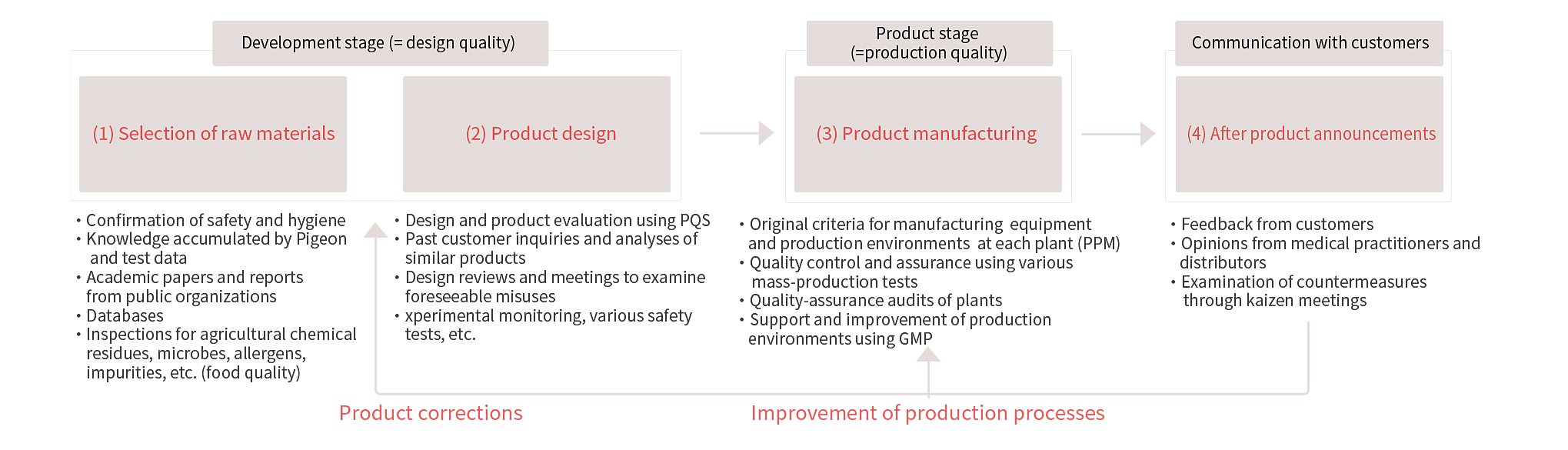
Selection of Safe Raw Materials
We use only raw materials that are confirmed to be safe from the product development stage.
Products Such as Nursing Bottles and Pacifiers
All plastics and rubber materials used in Pigeon nursing bottles, pacifiers, breast pumps and similar products are made using only raw materials that are confirmed to be safe and hygienic. For all of the raw materials it uses, we confirm information from inside and outside sources and assesses safety-related risks, understanding the physical and chemical properties of each and checking whether they contain harmful substances. In addition, we employ outside organizations to conduct safety tests, using evaluation methods stipulated in Japanese and overseas public qualification criteria, thereby ensuring objective evaluation.
Skincare Products, Shampoos, etc.
We confirm internal and outside information regarding the ingredients used in skincare products, lotions, bottom wipes and so on, and conducts assessments of the related safety risks. In addition, the Group enables widespread use of the knowledge and testing data it has amassed through past raw-material evaluations, not only ourinternal information but also in academic papers, reports published by public organizations, databases and other outside information sources.
Baby Foods, etc.
We rigorously check the condition of its quality control on substances that affect food safety, such as agricultural chemical residues, microbes, allergens and impurities, adopting only raw materials that are safe and of high quality. We have devised its systems so that all information on the raw materials used in products is managed via a database, enabling prompt and accurate provision of information when customer inquiries are received.
Product Design and Safety Confirmation
At the development and design stages, we us an original internal review system (including design review meetings, meetings to examine foreseeable misuses, etc.) to predict and analyze potential risks that may arise when products are in actual use, such as misuses, injuries and accidents. We then build measures against these risks into our product designs.
Wep evaluate and test design quality using experimental monitor tests. These tests examine whether products under development are built with the necessary quality to meet customer needs and whether improvements are needed.
Nursing Bottles, Pacifiers, etc.
We confirm the safety of its products through a battery of procedures at the development stage. These include reviews through original Pigeon systems for review of development processes (such as design review meetings and meetings to examine foreseeable misuses), comparative analysis with competitor and similar products, and experimental monitor tests. These tests enable us to perform multifaceted, objective evaluation of measures to prevent any types of failures and the feeling of using each product. By applying product design according to PQS across its global platform, we work hard to support and improve the design quality of the products it sells in countries worldwide.
Skincare Products, Shampoos, etc.
We conduct a wide range of tests to confirm product safety.
We employ a wide variety of testing approaches. We conduct tests using cells, tests by placement on human skin (such as human patch tests), component analysis to confirm whether harmful ingredients are included in a material or product, and time-lapse tests to confirm changes in characteristics over time. In addition to these,we use an original internal review system (including design review meetings, meetings to examine foreseeable misuses, etc.) to reflect the details of previous customer inquiries into products. Also, experimental monitor tests are used to examine function and uncover points for improvement, thereby confirming design quality.
Baby Foods, etc.
To ensure the safety of Pigeon food products, Wescrupulously check the shape and physical properties of foods at the development and design stages. We strive to minimize risk by confirming quality stability through storage tests and analyzing from multiple perspectives whether product contents, containers, packaging, etc. could cause injuries or accidents during use. Moreover, tWe also conduct regular audits of all cooperating manufacturers to whom it entrusts production and works continually with all cooperating manufacturers to support quality control systems at required levels and promote improvements.
During Product Manufacturing
Our products are manufactured only at plants that satisfy the manufacturing environment stipulated by us. We regularly conduct QA audits of production sites from a variety of angles, to uphold quality at each plant.
Products manufactured at these sites are tested at every stage. Only products that pass inspections from acceptance inspection of materials to process inspection during production and shipping inspection of the final product are shipped to customers.
In the event that a customer reports a problem originating in the manufacturing process or make a request, feedback is immediately provided to the production side. Manufacturing processes are then examined to determine whether corrections can be made.
Communication with Customers (After Product Launches)
Once a product is launched, we gather market information (such as feedback from customers and inquiries from medical facilities). We then build a framework for reflecting that information into safety evaluations of materials and products.
In the event that a suspected safety problem arises, the related sectors hold review meetings (kaizen meetings) to elucidate the causes of the problem and devise necessary countermeasures in a timely manner.
At the Pigeon Group, safety is always uppermost in our minds. The Group evaluates and tests its activities from the customer’s perspective, supporting safety and quality at exacting levels.
Our Policy on Animal Testing
The Pigeon Group recognizes the movement to eliminate animal testing as a vital global demand. We are undertaking an array of measures aimed at achieving the elimination of animal testing.
We never conduct tests using animals in the development of cosmetics (including quasi-drugs) and never permits its outside contractors to do so. Moreover, hawse have no plans to conduct or permit such tests in the future1.
We use original criteria to secure the safety of its cosmetics. Our approach is to use the knowledge about safety we have accumulated on various formulations and ingredients from previous development activities and to conduct tests on substitutes for animal testing and other solutions.
On substitutes for animal testing, we continuously collect information in partnership with industry organizations, academic societies and other companies, in Japan and overseas. In this way we actively adopt testing methods of high reliability listed in international testing guidelines.
1: This statement does not include cases where such testing unavoidable due to a duty to explain product safety to the public or government mandating of testing in certain countries. Even when we conduct animal testing in these unavoidable circumstances, we make every effort to minimize animal testing based on the basic philosophy of the three Rs of tender care: Replacement, reduction, refinement.